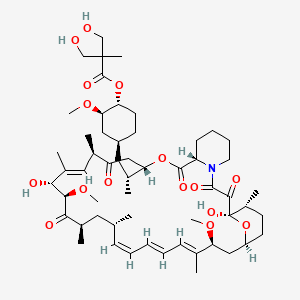
Temsirolimus / Temsirolimus Cas 162635 04 3 Chemsrc
Temsirolimus side effects. Temsirolimus is used in the treatment of advanced renal cell cancer.
Temsirolimus an inhibitor of mammalian target of rapamycin mTOR has proven beneficial in the treatment of advanced RCC with poor prognosis.

Temsirolimus. Temsirolimus is a type of targeted cancer drug treatment called an mTOR inhibitor. Drugs used in chemotherapy such as irinotecan hydrochloride and temozolomide work in different ways to stop the growth of tumor cells either by killing the cells or by stopping them from dividing. Temsirolimus comes as a solution liquid to be given by infusion slow injection into a vein over 30 to 60 minutes.
Temsirolimus also stops the cancer from making blood vessels which the cells need to be able to grow. Temsirolimus is also being studied in the treatment of other types of cancer. The TORISEL vial includes an overfill of.
Definition from the NCI Drug Dictionary - Detailed scientific definition and other names for this drug. It is not yet known whether combination chemotherapy is more effective when given together with bevacizumab or temsirolimus in treating rhabdomyosarcoma. Among 331 eligible treated patients median PFS was 75 months for bevacizumab alone 90 CI 58 to 108 months 76 months for bevacizumab plus temsirolimus 90 CI 67 to 92 months 92 months for bevacizumab plus sorafenib 90 CI 75 to 114 months and 74 months for sorafenib plus temsirolimus 90 CI 56 to 79 months.
3 DOSAGE FORMS AND STRENGTHS TORISEL temsirolimus is supplied as a kit consisting of the following. Some side effects may occur during the injection. Temsirolimus is an inhibitor of mTOR mammalian target of rapamycin.
Temsirolimus Injection requires two dilutions prior to intravenous infusion. MTOR is a serinethreonine kinase which plays a role in the PI3KAKT pathway that is upregulated in some tumors. Temsirolimus is degraded by both acids and bases and thus combinations of temsirolimus with agents capable of modifying solution pH should be avoided.
It is usually given by a doctor or nurse in a doctors office or infusion center. It blocks the effects of a protein called mTOR that is often over active in cancer cells. Pfizer RxPathways connects eligible patients to a range of assistance programs that offer insurance support co-pay help and medicines for free or at a savings.
You may experience symptoms such as. Temsirolimus is an ester analog of rapamycinTemsirolimus binds to and inhibits the mammalian target of rapamycin mTOR resulting in decreased expression of mRNAs necessary for cell cycle progression and arresting cells in the G1 phase of the cell cycle. Temsirolimus induces autophagy and apoptosis.
This protein makes the cells divide and grow. Temsirolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Temsirolimus is approved to treat.
TORISEL Quick Finder. Temsirolimus is usually given once every week. Sellecks Temsirolimus CCI-779 has been cited by 77 publications.
Tell your caregiver right away if you feel dizzy warm. What Temsirolimus Is Used For. Temsirolimus is a targeted therapy and is classified as a mTOR inhibitor.
Temsirolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. The primary aim of this clinical trial was to prioritize bevacizumab or temsirolimus for additional investigation in rhabdomyosarcoma RMS when administered in combination with cytotoxic chemotherapy to patients with RMS in first relapse with unfavorable prognosis. Temsirolimus CCI-779 NSC 683864 is a specific mTOR inhibitor with IC50 of 176 μM in a cell-free assay.
Temsirolimus Injection should be diluted only with the supplied DILUENT for Temsirolimus Injection. TORISEL temsirolimus injection 25 mgml. Renal cell carcinoma a type of kidney cancer that is advanced.
Patients were randomly assigned to receive bevacizumab on day 1 or temsirolimus on days 1 8 and. Get emergency medical help if you have signs of an allergic reaction hives difficult breathing swelling in your face or throat or a severe skin reaction fever sore throat burning eyes skin pain red or purple skin rash with blistering and peeling. If a drug has been approved for one use physicians may elect to use this same drug for other problems if they believe it may be helpful.
Temsirolimus Injection 25 mgmL is a clear colorless to light yellow non-aqueous ethanolic sterile solution. 990 rows Mechanism of action. The rationale for mTOR inhibitors in treatment of RCC the pharmacokinetics and toxicities of temsirolimus landmark clinical trials of temsirolimus in advanced RCC and the indications for its use in.
Temsirolimus CCI-779 is an inhibitor of mammalian target of rapamycin mTOR kinase a component of intracellular signaling pathways involved in the growth and proliferation of cells 1011 and.
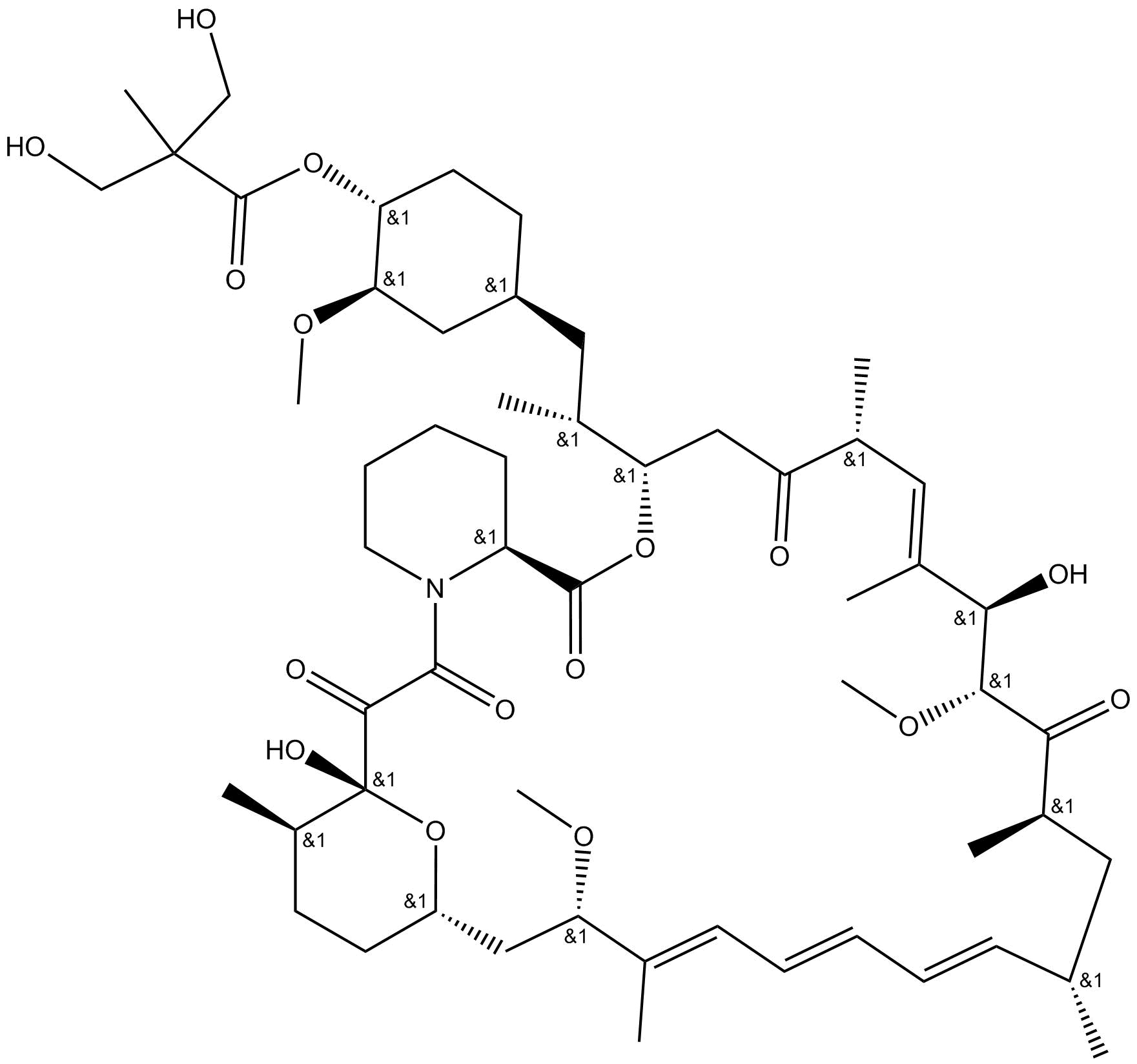
Apexbio Temsirolimus Mtor Inhibitor Cas 162635 04 3
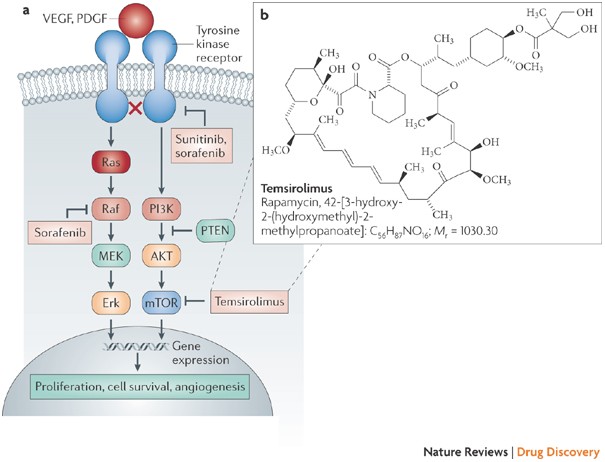
Temsirolimus Nature Reviews Drug Discovery

File Temsirolimus Png Wikipedia

Temsirolimus An Inhibitor Of Mammalian Target Of Rapamycin Clinical Cancer Research

Temsirolimus 162635 04 3 Reference Standards Alsachim
Temsirolimus Injection Now Available From Fresenius Kabi Business Wire
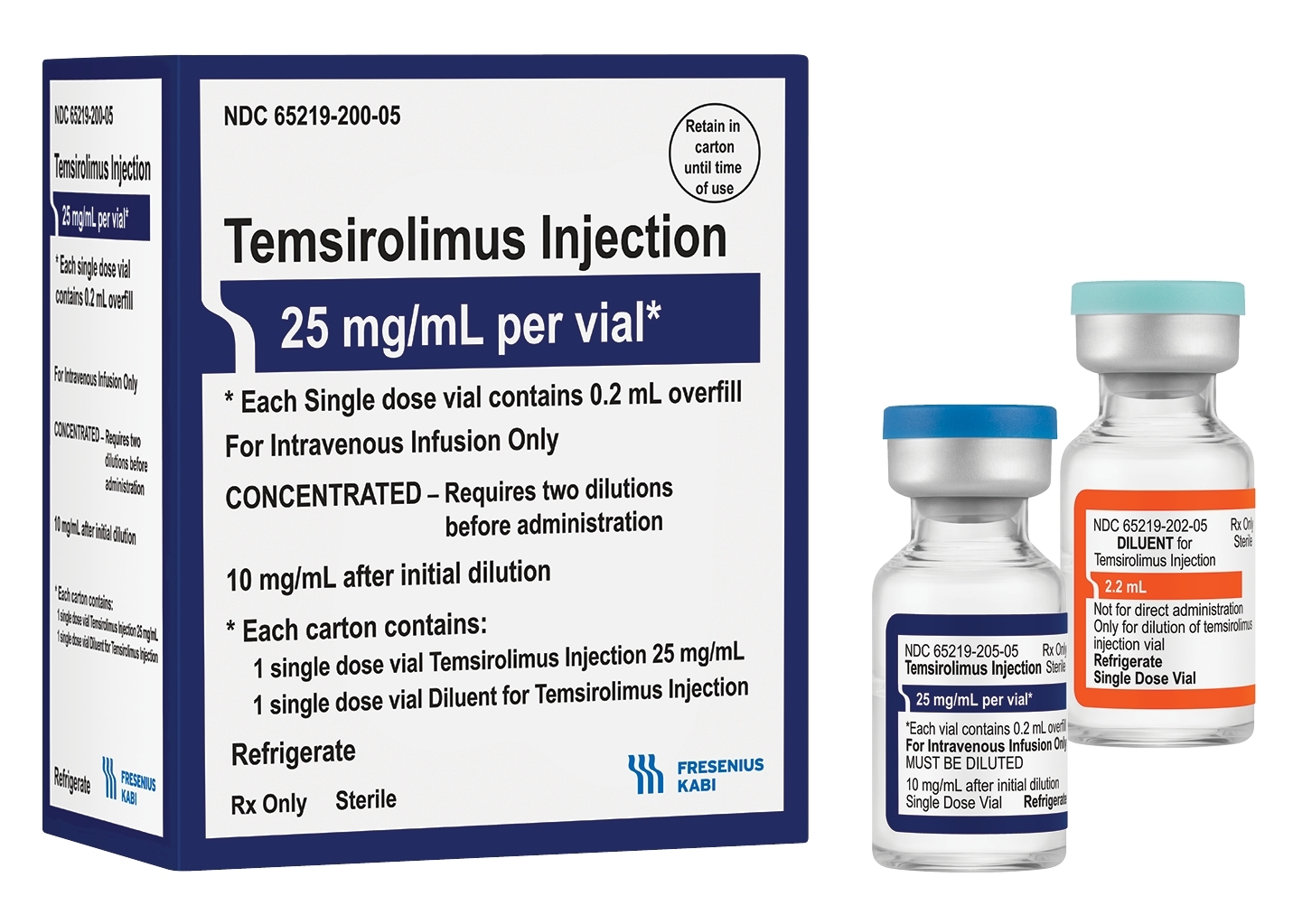
Temsirolimus Injection Now Available From Fresenius Kabi Business Wire

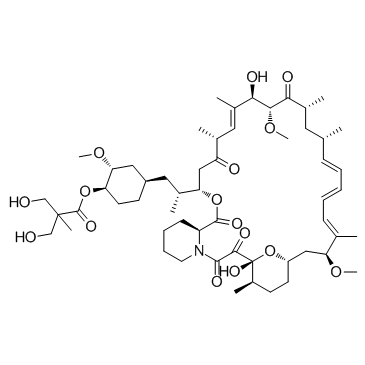
Structure Of Temsirolimus Download Scientific Diagram

Temsirolimus 98 Hplc 162635 04 3
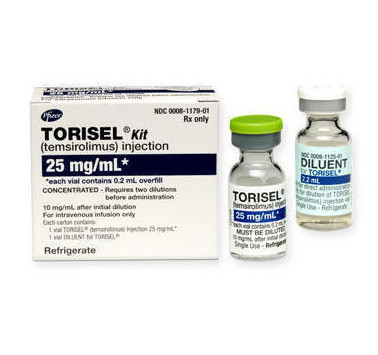
Torisel Temsirolimus Cancer Medication Cancer Health
Temsirolimus Cas 162635 04 3 Chemsrc

A10906 10 Temsirolimus 162635 04 3 Clinisciences
Temsirolimus C56h87no16 Pubchem

Temsirolimus 162635 04 3 Tci Europe N V

Temsirolimus 162635 04 3 Tci Europe N V
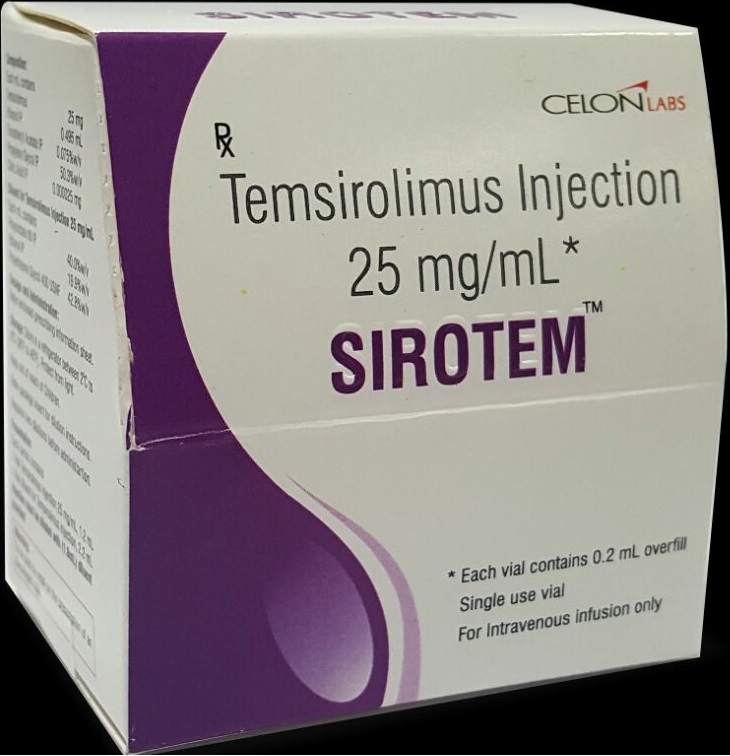
Sirotem Temsirolimus By Celon India Generic Version Of Torisel